Samospharma Challenges FDA Ghana with Port Documents in Ongoing Opioid Scandal
- In intercepted documents, Samospharma’s response to FDA
The pharmaceutical industry in Ghana is facing a fresh controversy as NorvanReports has stumbled on new documents in which Samospharma Limited has publicly challenged FDA Ghana’s directive, presenting port declaration documents as evidence to dispute claims that it engaged in the illegal importation of opioids.
The company has also issued a categorical and complete dissociation from Aveo Pharmaceuticals Pvt. Ltd. and Westfin International Pvt. Ltd., stating that it has never worked with them in any capacity.
The company’s latest move marks a significant escalation in the dispute following allegations raised by BBC Africa Eye that pharmaceutical companies, including Samospharma, had been involved in the influx of unregulated opioids into West Africa.
Samospharma, however, has maintained its innocence and is now presenting customs records to back its claims in a letter to the FDA Ghana, a letter date February 25, 2025, addressed to the regulartor through its Chief Executive Officer and sighted by the NorvanReports.
Samospharma’s Core Defense: Port Documentation
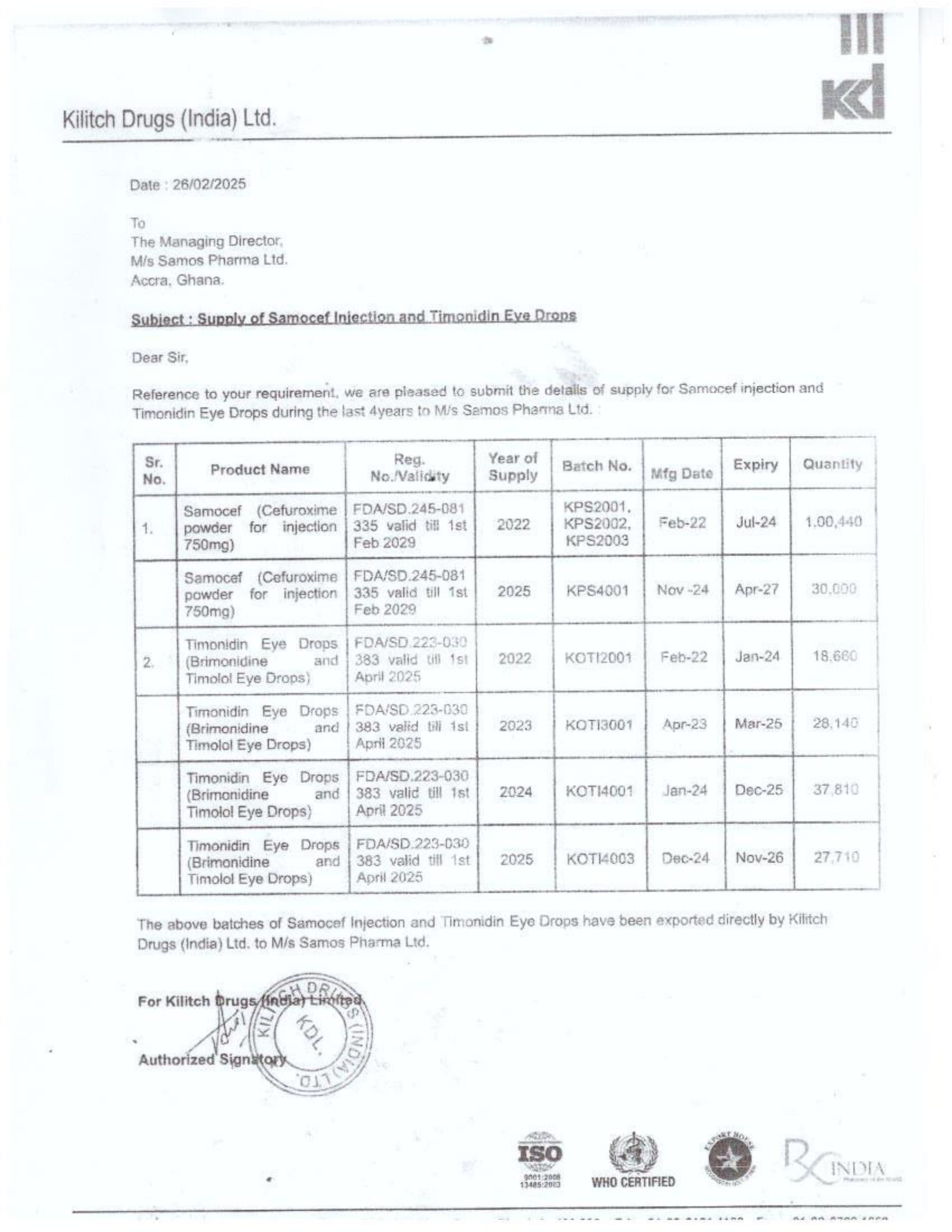
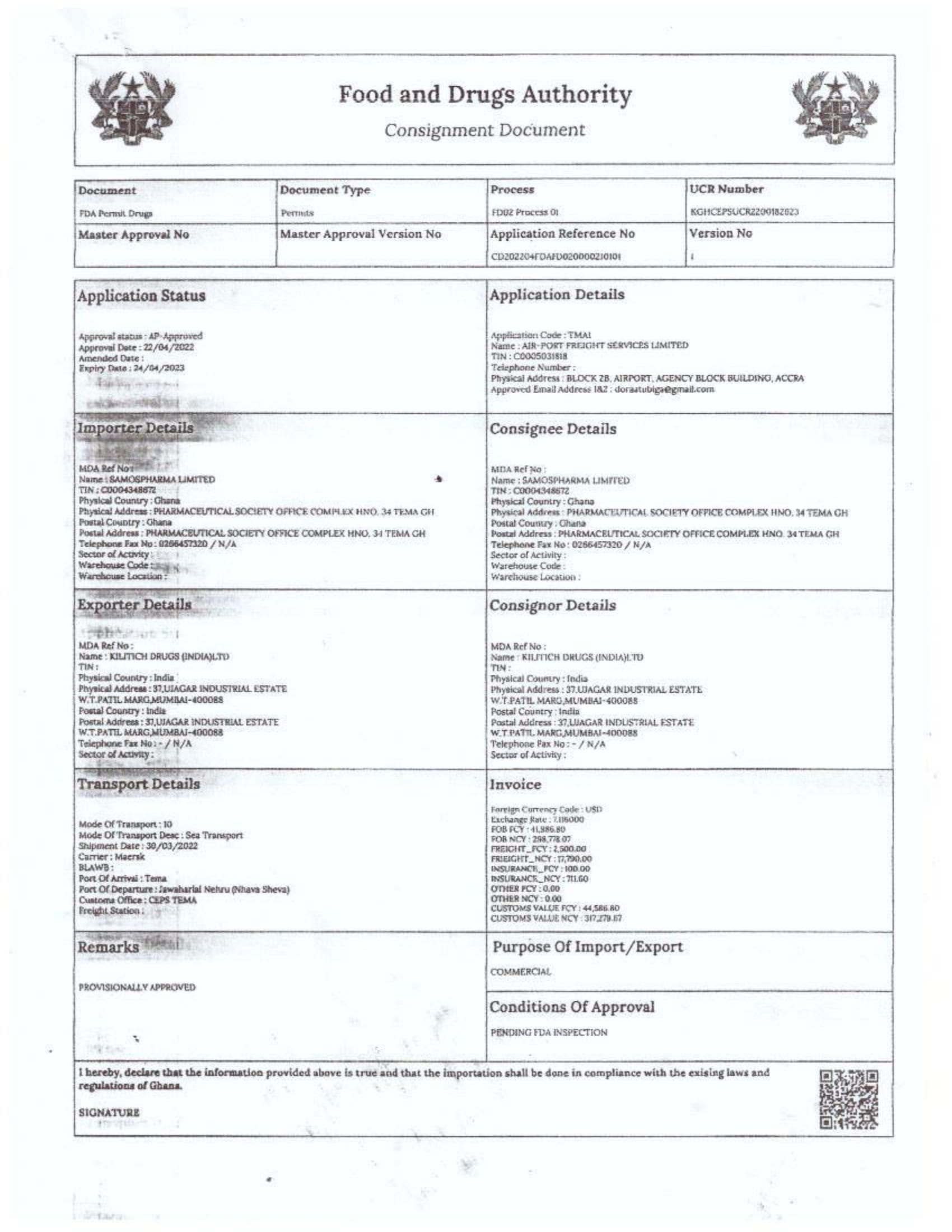
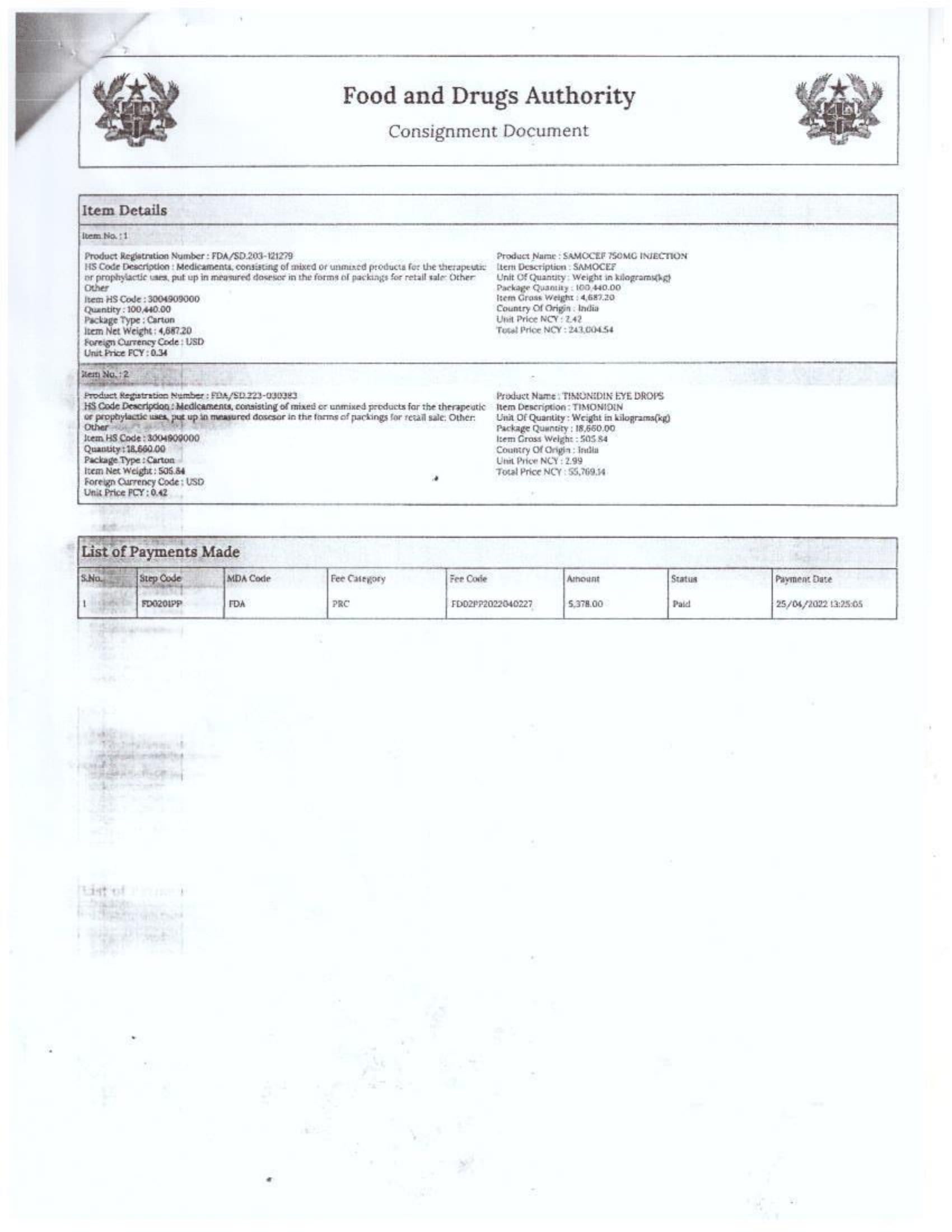
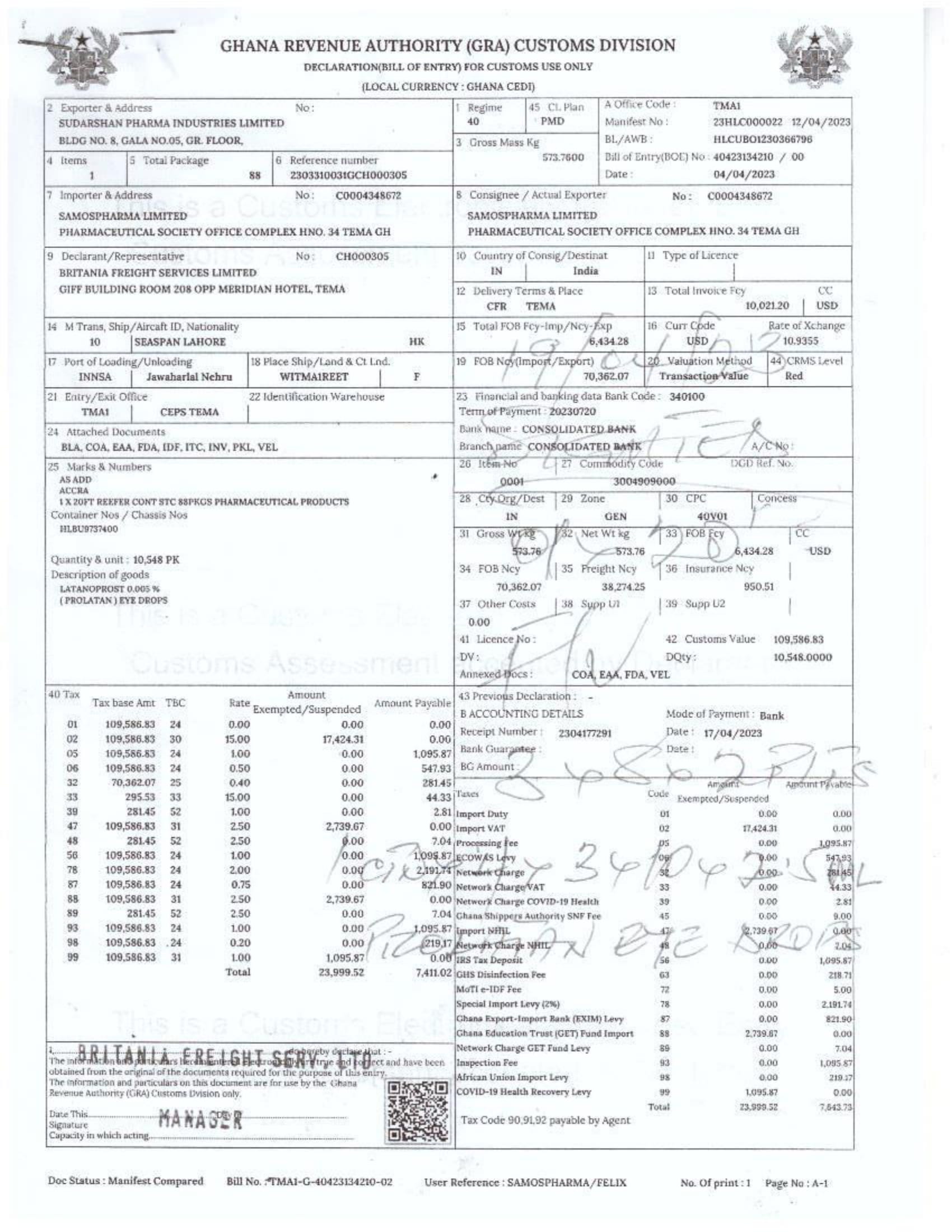
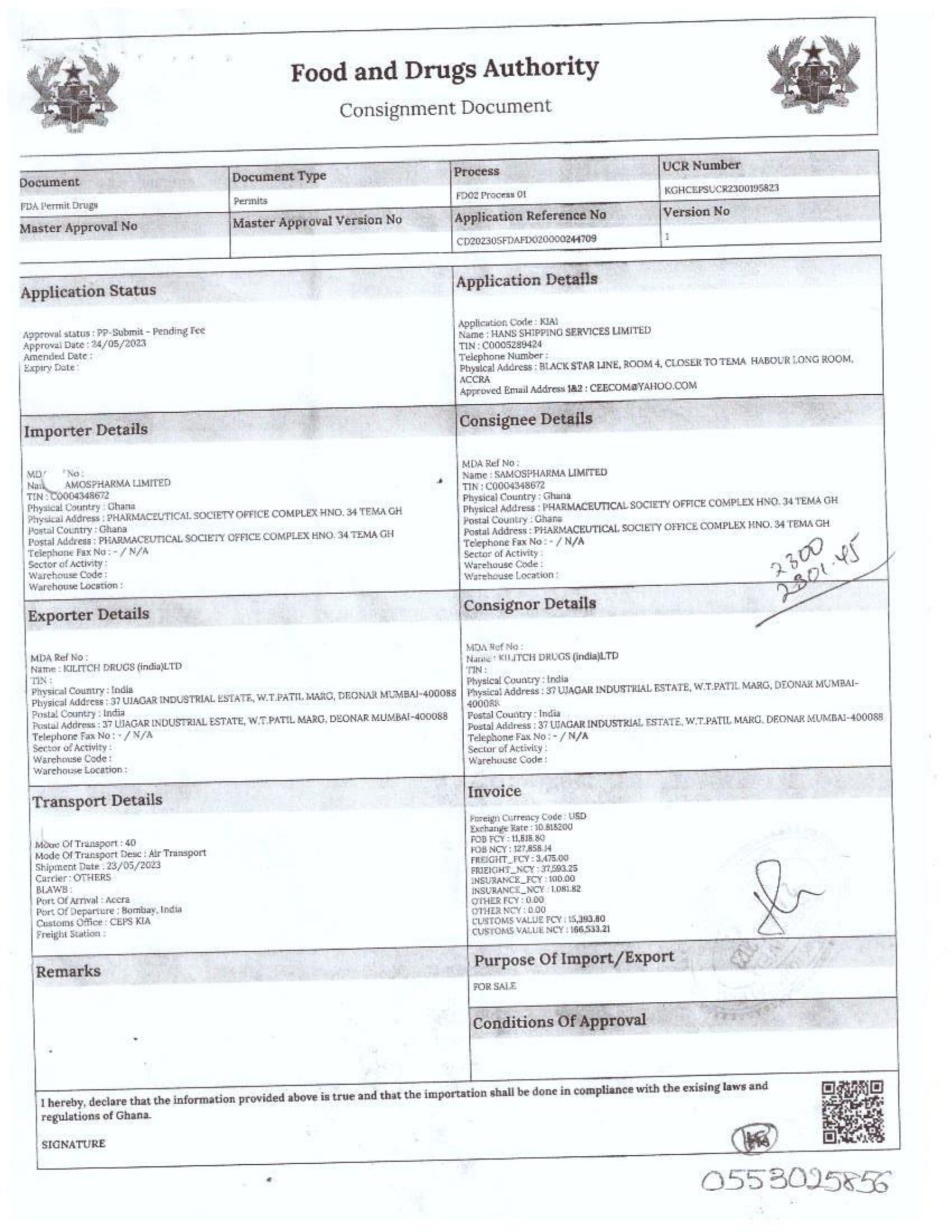
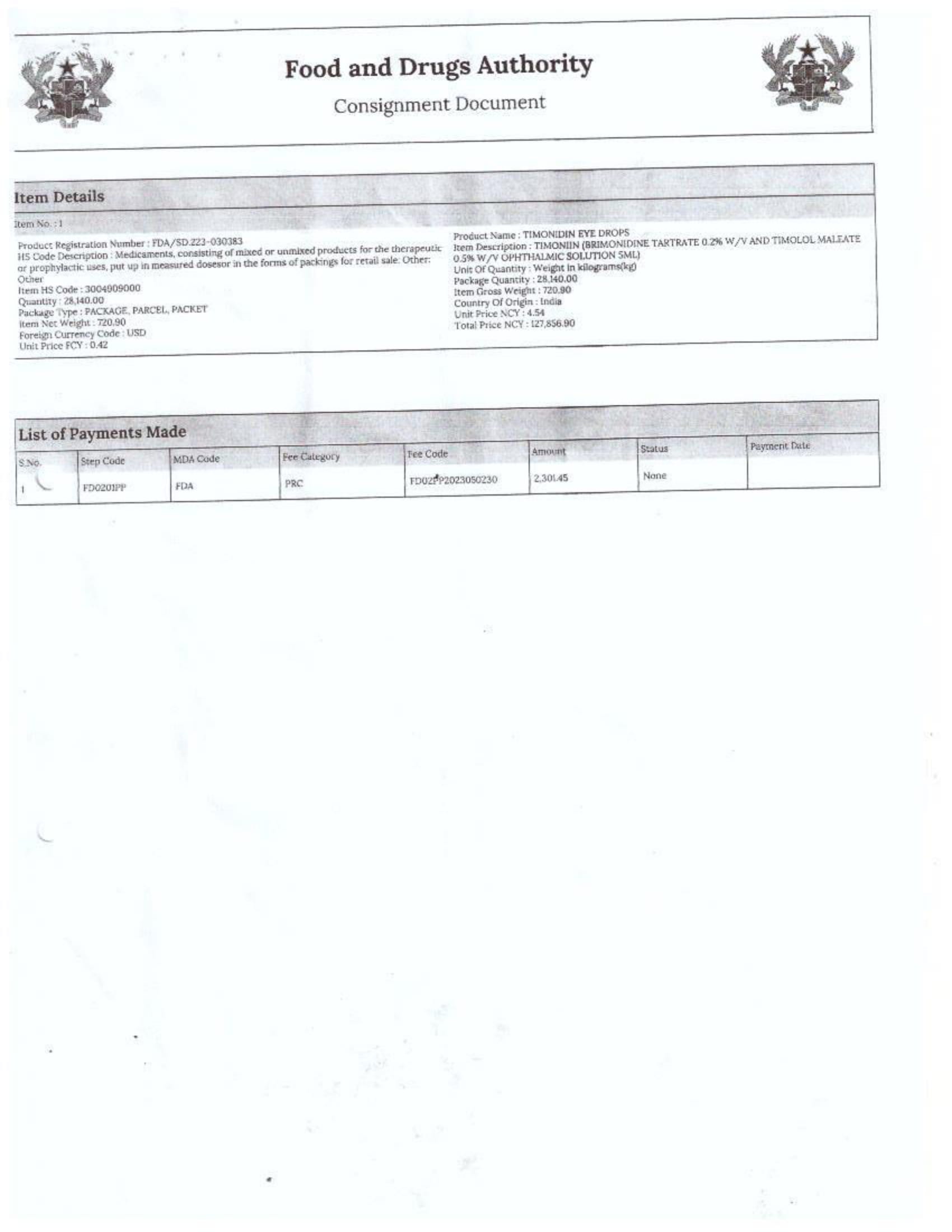
In response to FDA Ghana’s February 2025 directive, which instructed the company to cease imports from Aveo Pharmaceuticals Pvt. Ltd. and Westfin International Pvt. Ltd., Samospharma has provided official Tema Port declaration documents showing that:
- Every shipment processed through the port was properly documented and approved by relevant regulatory agencies.
- None of the declared imports included opioids such as tapentadol or carisoprodol.
- Customs clearance followed due process, making it impossible for the company to have smuggled in unapproved medications.
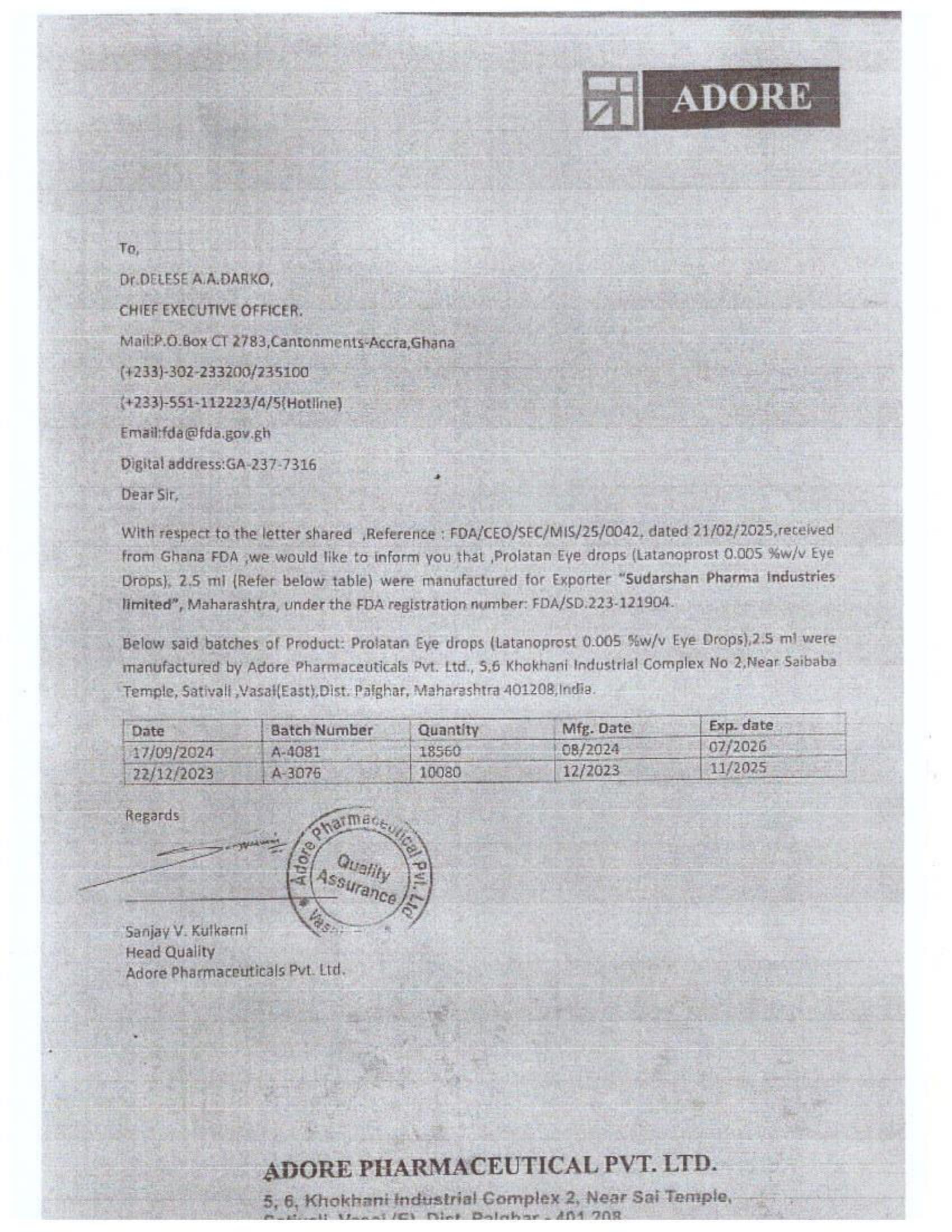
- The attached port declaration documents from Samospharma show entirely different company names, with no mention of Aveo Pharmaceuticals Pvt. Ltd. or Westfin International Pvt. Ltd., further proving that Samospharma has not conducted business with these companies.
- Samospharma has never worked with Aveo, Westfin, PRG Pharma, or any of their affiliates—whether as manufacturers, exporters, middlemen, or in any other capacity.
- The company categorically states that it was unaware of Aveo, WestFin, or PRG’s existence until the FDA’s recent directive tried to associate them.
“We have submitted full customs and port declarations that prove we have not imported any unregulated or illicit substances. This is clear evidence that the allegations against us are baseless and misleading. More importantly, we completely dissociate ourselves from Aveo, WestFin, and PRG—we have never worked with them in any capacity, and our documents prove it,” Samospharma stated in its response.
Contradictions in Regulatory Oversight: Who is Accountable?
The FDA’s order to halt further imports from the Indian exporters came after official records from 2022 and 2023 revealed that Samospharma had conducted business transactions with Aveo Pharmaceuticals and Westfin International. However, the company’s latest evidence raises serious concerns about regulatory inconsistencies, such as:
- Why did the FDA approve and clear previous shipments from these suppliers if they were deemed unfit?
- If all imports were legally declared at the port, how did regulatory agencies fail to detect any discrepancies earlier?
- What specific evidence does the FDA hold against Samospharma to justify its import ban?
- Why is the FDA trying to associate Samospharma with companies it has never worked with, based on no evidence?
A Shift in Strategy: Samospharma Demands Retraction
Samospharma’s latest response takes an aggressive turn, calling for a retraction of FDA Ghana’s statement and demanding a review of the import ban.
“We expect the regulatory bodies to acknowledge this evidence and rectify their statements accordingly. We will not allow our reputation to be tarnished by unverified allegations. The FDA must provide clear proof of how they linked us to these companies, or they must retract their statement immediately,” Samospharma declared.
The company further indicated that it would consider legal action if the regulatory body fails to publicly acknowledge the customs evidence.
Regulatory Fallout: Questions for Ghana’s FDA
The battle between Samospharma and FDA Ghana now raises larger questions about transparency, accountability, and due process in the country’s pharmaceutical regulatory framework. If Samospharma’s port documentation is accurate, the FDA’s actions could be perceived as reactionary rather than based on concrete evidence.
Key concerns include:
- Has FDA Ghana mismanaged the enforcement of import regulations, leading to wrongful accusations?
- Should regulatory agencies conduct an independent review of import controls at Tema Port to verify whether other pharmaceutical companies face similar issues?
- What measures will be taken to ensure that future regulatory actions are evidence-based rather than reactionary?
- Why was Samospharma linked to Aveo and WestFin without clear evidence?
Industry-Wide Implications and Potential Legal Battles
As the pharmaceutical sector watches this dispute unfold, Samospharma’s response has set the stage for potential legal showdowns, not just with regulators but also with media houses that reported the original allegations.
The company has indicated that it may take legal action against BBC Africa Eye, which was the first to publicly link them to the alleged opioid trade. Additionally, if FDA Ghana does not retract its directive, the company could pursue litigation to protect its business interests.
What Comes Next?
The ongoing battle between Samospharma, FDA Ghana, and investigative media is likely to shape the future of pharmaceutical regulations in Ghana. With evidence now being contested publicly, there will be increasing calls for:
- A full-scale parliamentary or independent inquiry into how regulatory actions are taken and justified.
- A more transparent system for tracking pharmaceutical imports, with digital records accessible for verification.
- Greater accountability for both importers and regulators, ensuring that due process is followed at all stages.
For now, Samospharma is taking a defiant stand, challenging the credibility of regulatory actions against it. As the dispute unfolds, the credibility of Ghana’s pharmaceutical oversight framework is now under global scrutiny.
Find below the documents intercepted by the NorvanReports.