SONA 2025: President Mahama Awaits Probe Findings on Aveo Opioid Scandal, Pledges Action on Findings
President John Dramani Mahama has stated that his administration is awaiting the outcome of ongoing investigations into the controversial opioid case involving Ghanaian-owned pharmaceutical company Samospharma Limited and two India-based firms, Aveo Pharmaceuticals Pvt Ltd. and Westfin International Pvt. Ltd.
Delivering his first State of the Nation Address (SONA) on Thursday, February 27, 2025, Mahama assured that the findings of the investigations would be made public, with appropriate measures taken.
“We await investigations into the opioid case; outcomes will be shared, and actions will be taken,” he affirmed.
The case follows a BBC Eye Africa report highlighting a rising opioid crisis in West Africa, with Ghana emerging as a key entry point for the unregulated and highly addictive drugs. The report revealed that shipments of illicit opioids are entering the region not through smuggling networks but via legal import channels, raising concerns over regulatory oversight.
At the center of the scandal is Indian pharmaceutical entrepreneur Vinod Sharma, whose company, Aveo Pharmaceuticals, has allegedly been exporting unapproved opioids—including brands such as Timaking, Tafrodol, and Super Royal-225—into Ghana and Nigeria. The report claims that these shipments lack regulatory approval anywhere in the world.
Documents cited by BBC Eye Africa further allege that Ghana’s largest opioid importer is Samospharma Limited, which by November 2024 had imported over $6 million worth of opioids from Indian manufacturers, including PRG Pharma.
The report also noted that Samospharma’s leadership includes prominent figures in Ghana’s pharmaceutical industry, some of whom have advised the government on drug regulation and e-pharmacy policies.
Samospharma Denies Allegations
In response, Samospharma Limited has strongly refuted the allegations, categorically denying any involvement in the illegal trade of tapentadol, carisoprodol, or similar substances.
“The claims are defamatory, baseless, and a serious act of criminal impersonation,” the company stated.
Samospharma also criticized the BBC’s reporting, stating that “at no point did the BBC/BBC Africa Eye enter the offices or facilities of Samospharma to ascertain the veracity of their claims, and this we find very scandalous and libelous.”
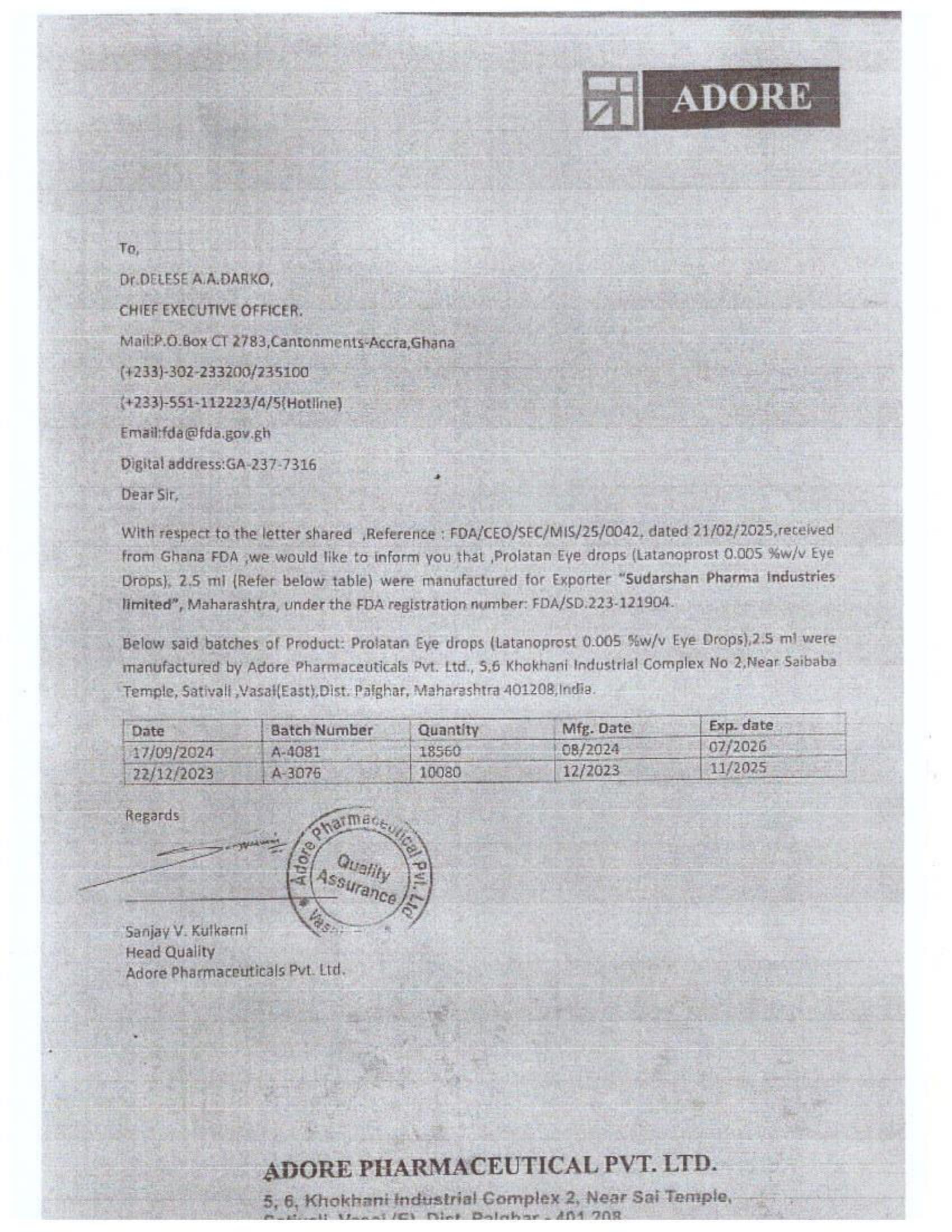
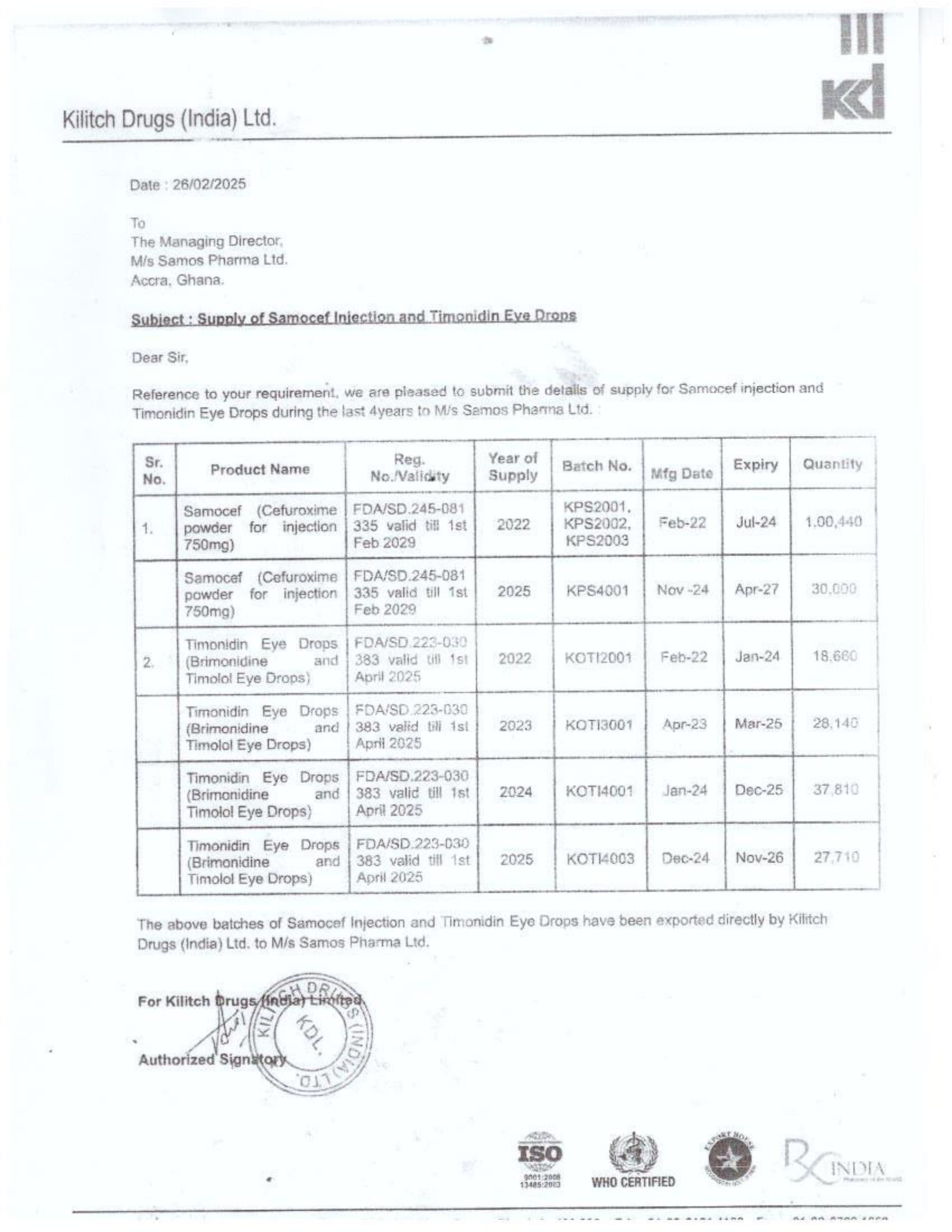
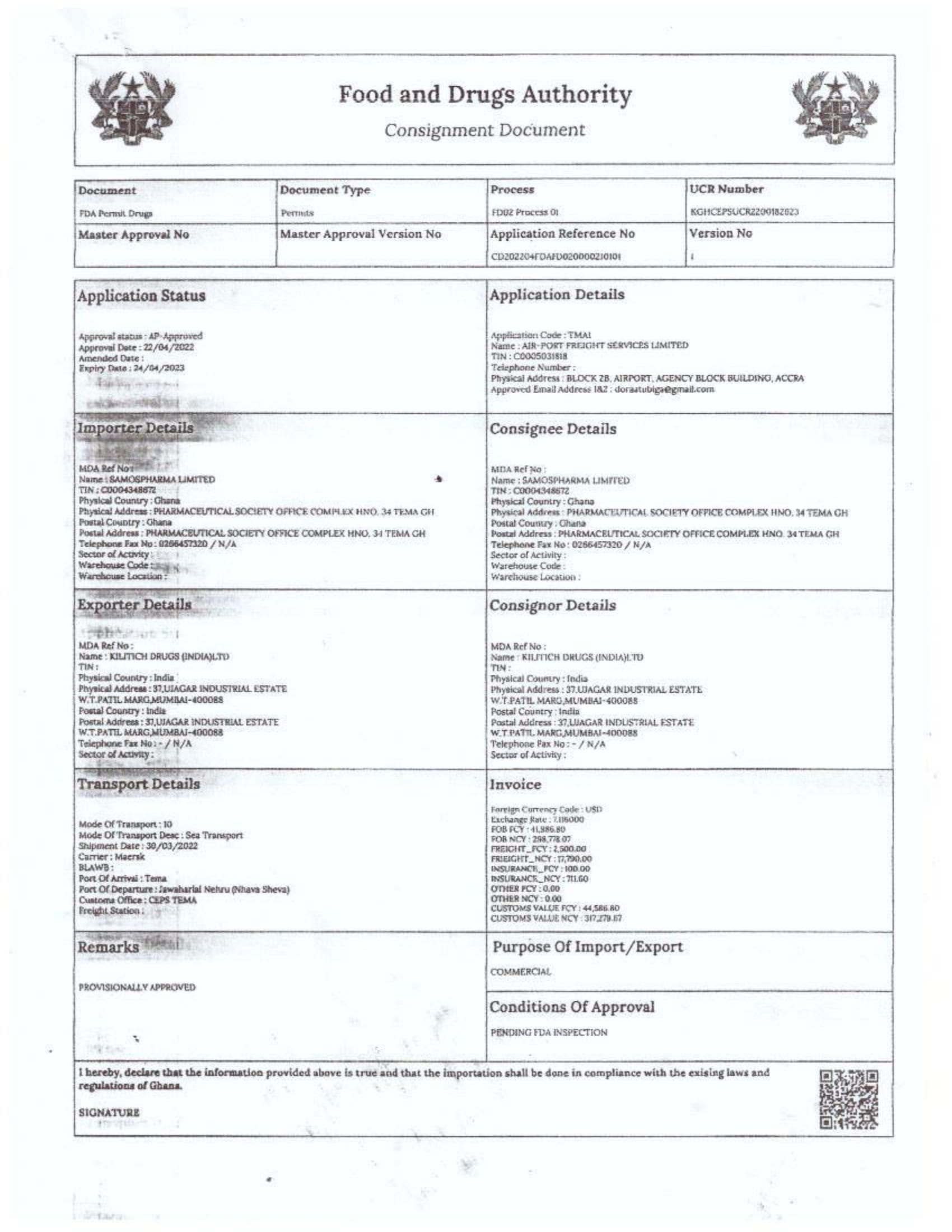
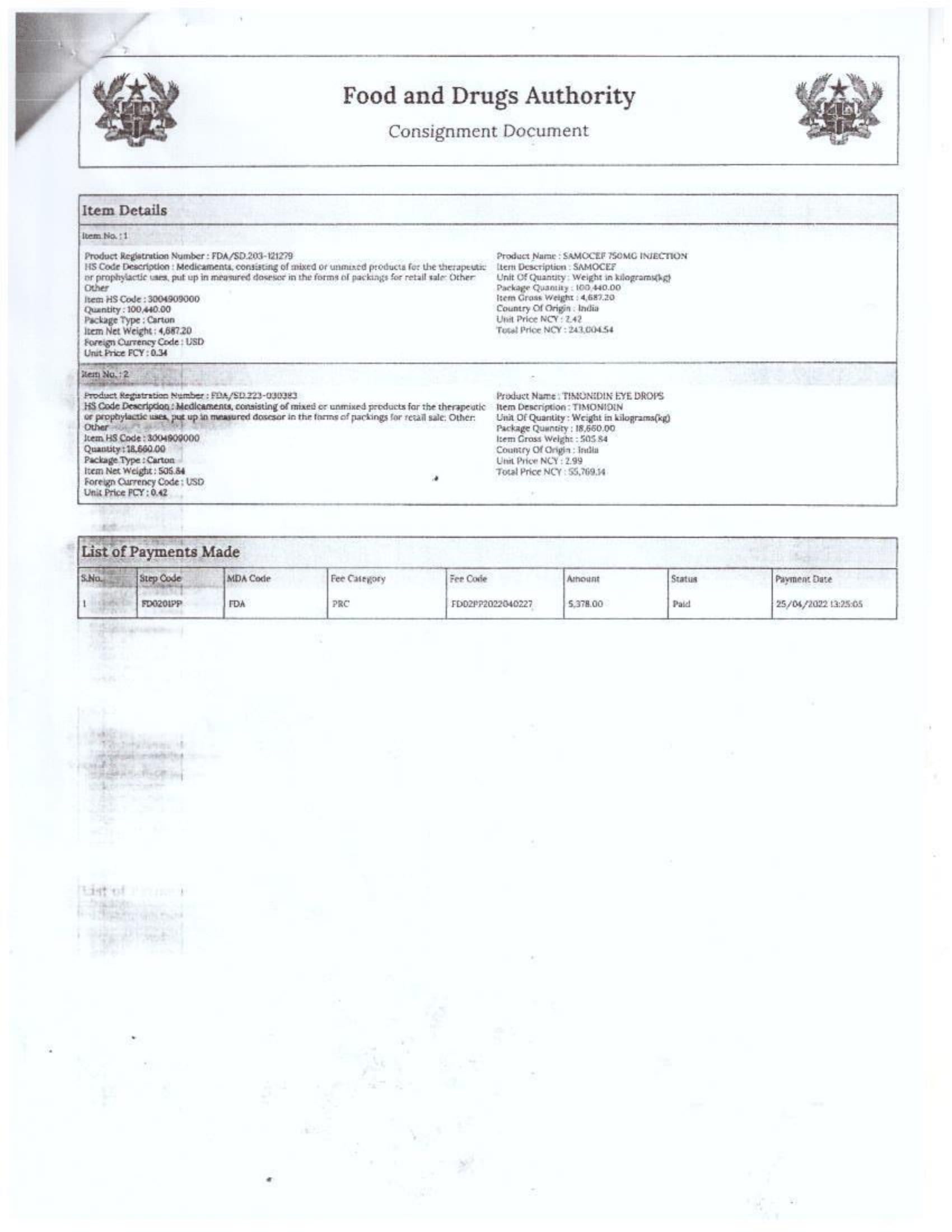
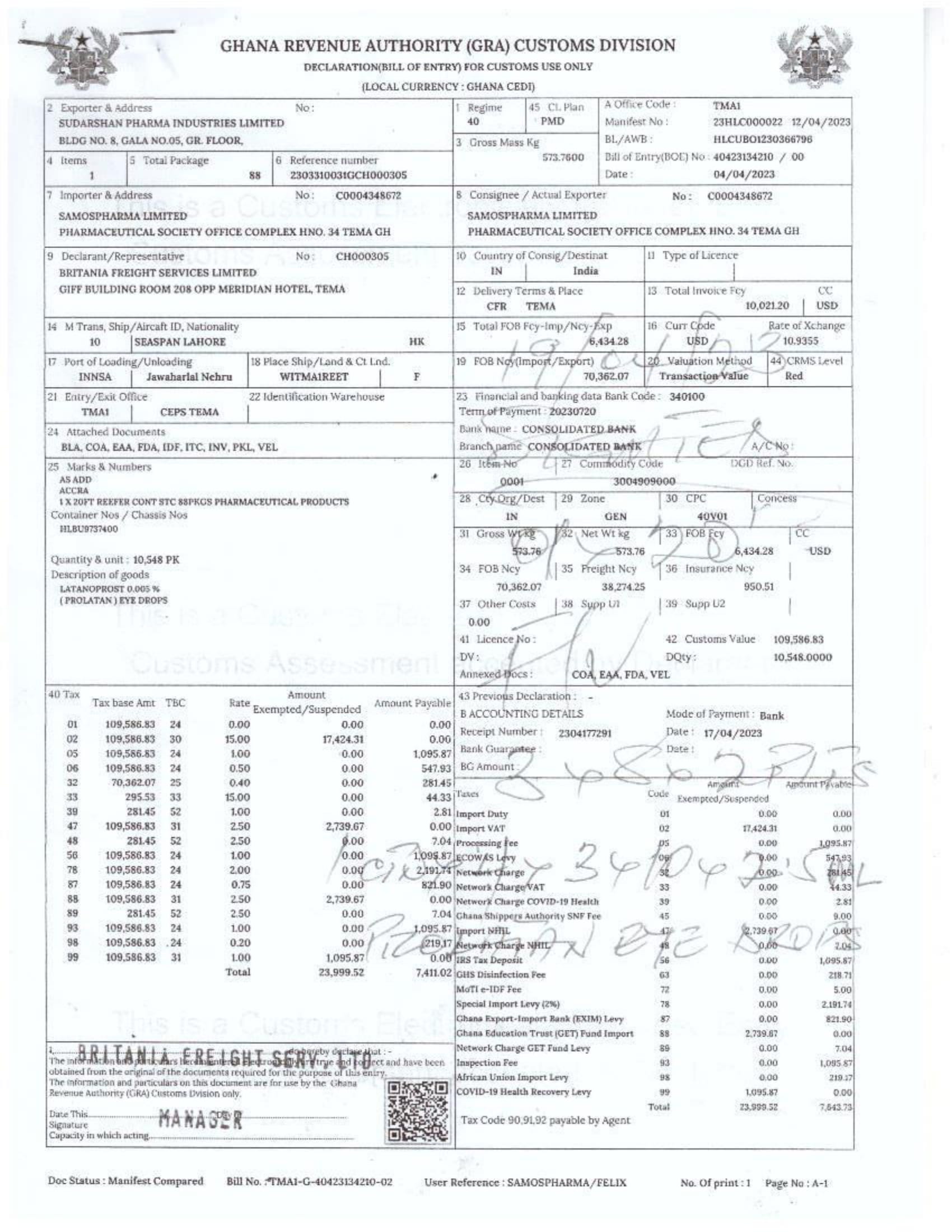
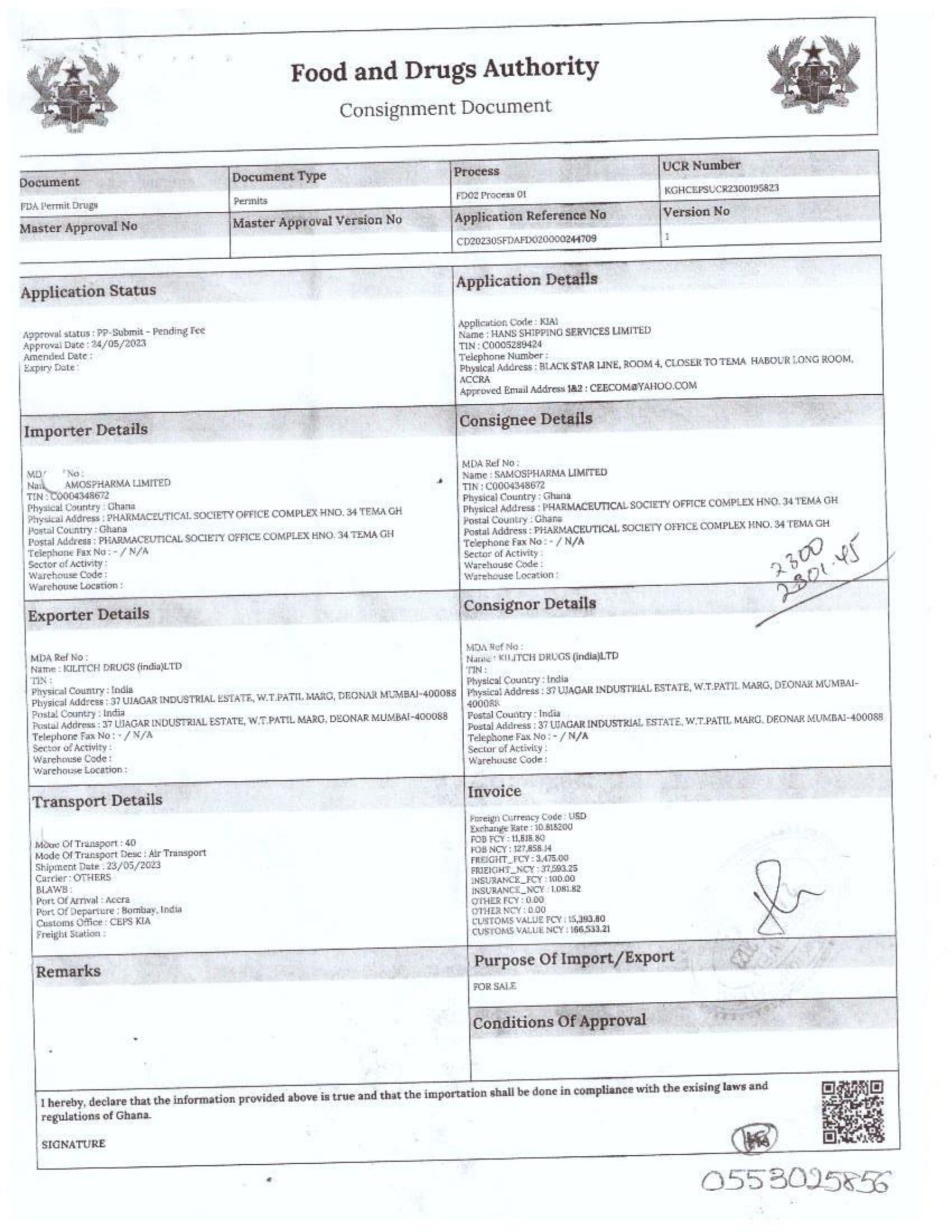
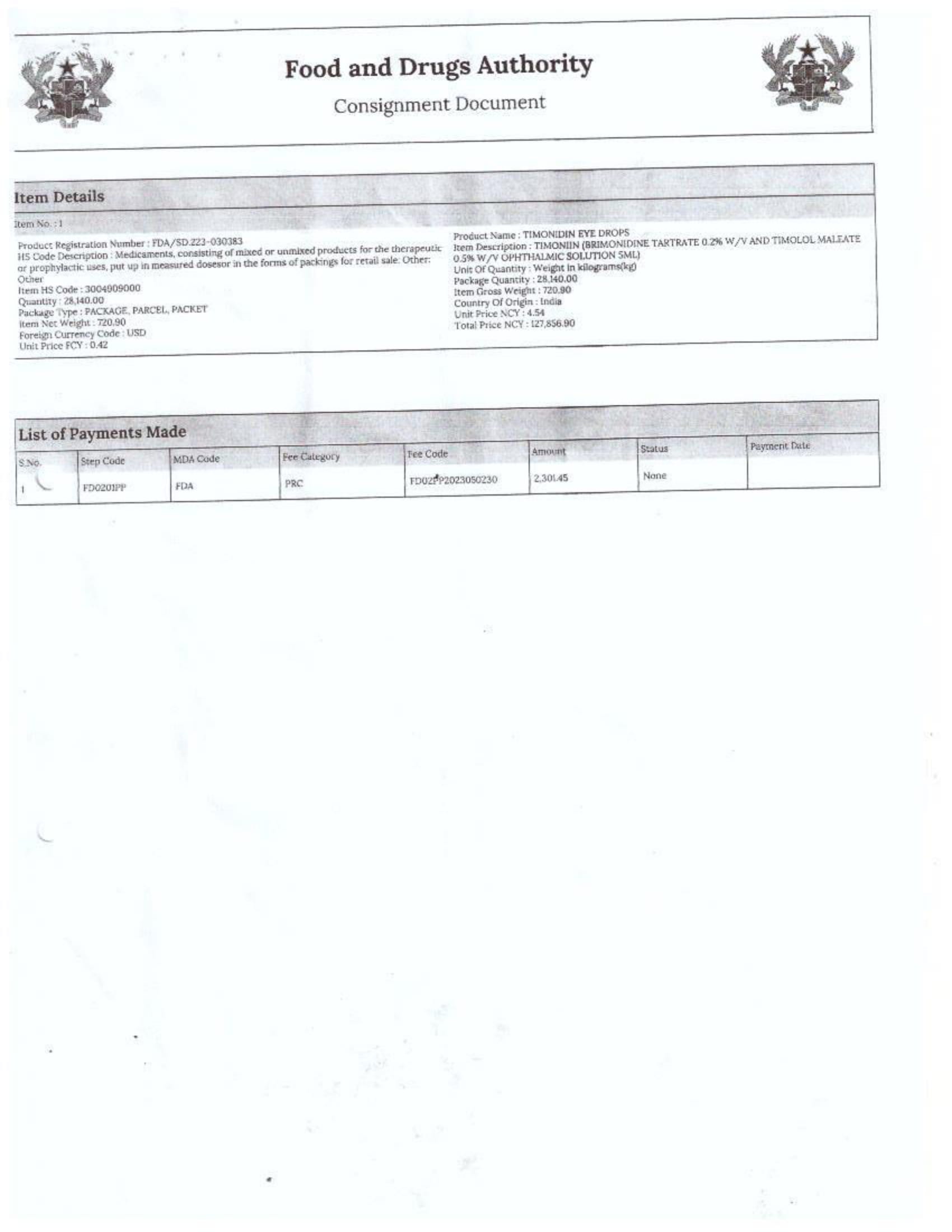
Menawhile NorvanReports has stumbled on new documents in which Samospharma Limited has publicly challenged FDA Ghana’s directive, presenting port declaration documents as evidence to dispute claims that it engaged in the illegal importation of opioids.
The company has also issued a categorical and complete dissociation from Aveo Pharmaceuticals Pvt. Ltd. and Westfin International Pvt. Ltd., stating that it has never worked with them in any capacity.
The company’s latest move marks a significant escalation in the dispute following allegations raised by BBC Africa Eye that pharmaceutical companies, including Samospharma, had been involved in the influx of unregulated opioids into West Africa.
Samospharma, however, has maintained its innocence and is now presenting customs records to back its claims in a letter to the FDA Ghana, a letter date February 25, 2025, addressed to the regulartor through its Chief Executive Officer and sighted by the NorvanReports.